How is Carbon Dioxide Transported in the Blood
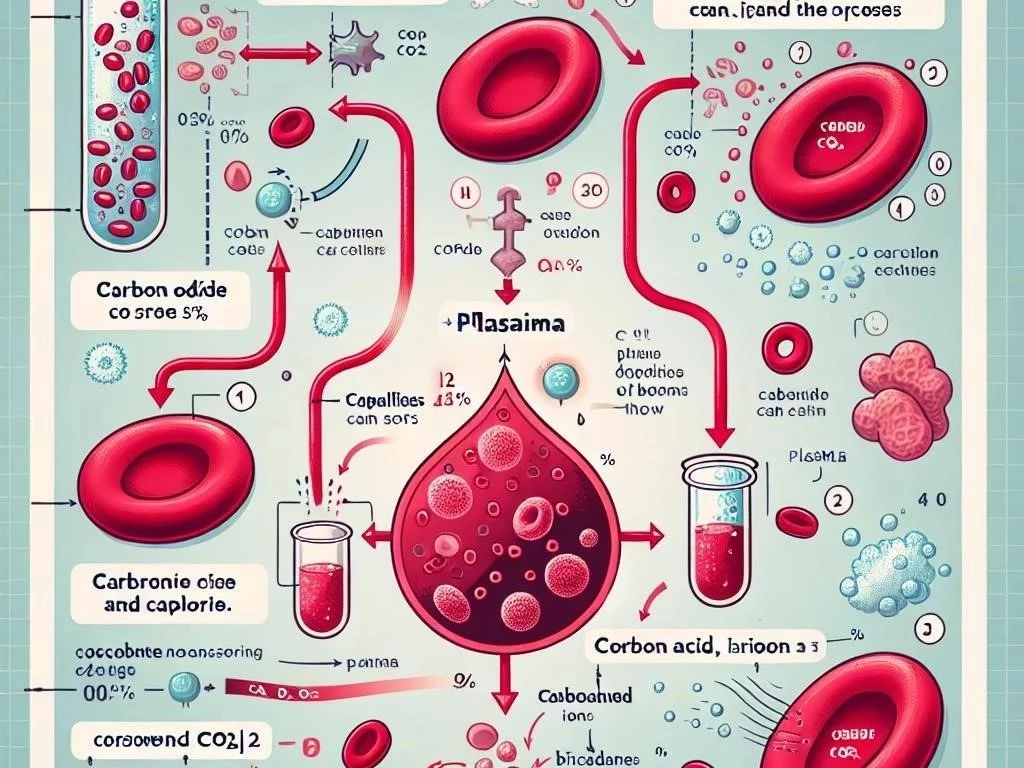
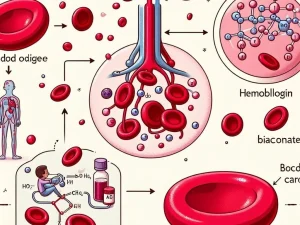
Carbon dioxide is transported in blood through three primary mechanisms: dissolved in plasma, converted to bicarbonate, and bound to hemoglobin in red blood cells.
Carbon dioxide transport is essential for maintaining acid-base balance, facilitating gas exchange, and removing metabolic waste produced during cellular respiration in the human body.
1.1 Importance of Carbon Dioxide in the Body
Carbon dioxide plays a critical role in the human body, primarily as a byproduct of cellular respiration. As cells metabolize nutrients for energy, they produce carbon dioxide, which must be efficiently transported to maintain homeostasis. Elevated levels of carbon dioxide can lead to respiratory acidosis, affecting the body’s acid-base balance. Furthermore, carbon dioxide is essential for stimulating breathing, as increased concentrations signal the respiratory system to enhance gas exchange rates. The transport mechanisms—dissolved in blood plasma, as bicarbonate ions, and bound to hemoglobin—ensure its effective removal from tissues. Understanding these processes is vital for recognizing how the body maintains optimal oxygen saturation and manages metabolic waste, contributing to overall health and well-being.
1.2 Overview of Blood Transport Mechanisms
The transport of carbon dioxide in the blood involves three primary mechanisms, ensuring efficient removal from tissues. First, a small portion of carbon dioxide dissolves directly in blood plasma, accounting for about 7-10% of total transport. The second mechanism involves the conversion of carbon dioxide into bicarbonate ions through the action of carbonic anhydrase, which facilitates a significant portion—approximately 70%—of carbon dioxide transport; Finally, around 20-23% of carbon dioxide binds to hemoglobin, forming carbamino compounds. This binding occurs primarily in venous blood, where carbon dioxide concentration is higher. Each mechanism plays a crucial role in maintaining acid-base balance and supporting efficient gas exchange during pulmonary circulation, ultimately contributing to optimal cellular respiration and metabolic function.
The Role of the Respiratory System
The respiratory system facilitates carbon dioxide removal through inhalation and exhalation, ensuring efficient gas exchange in alveoli while maintaining optimal oxygen and carbon dioxide levels.
2.1 Anatomy of the Respiratory System
The respiratory system is composed of several key structures, including the nasal cavity, pharynx, larynx, trachea, bronchi, and lungs. Air enters through the nasal cavity, where it is filtered, warmed, and humidified before passing through the pharynx and larynx. The trachea then divides into the bronchi, which branch into smaller bronchioles within the lungs. At the end of these bronchioles are the alveoli, tiny air sacs where gas exchange occurs. The alveolar walls are thin and surrounded by capillaries, facilitating the diffusion of oxygen and carbon dioxide. The diaphragm and intercostal muscles aid in breathing, expanding and contracting the thoracic cavity, ensuring air flows efficiently into and out of the lungs during respiration.
2.2 Function of the Alveoli in Gas Exchange
The alveoli are crucial for efficient gas exchange in the lungs, serving as the primary sites where oxygen and carbon dioxide are exchanged between air and blood. These tiny, sac-like structures are surrounded by a network of capillaries, facilitating the diffusion process. When oxygen-rich air enters the alveoli, oxygen passes through the thin alveolar walls into the blood, where it binds to hemoglobin in red blood cells. Simultaneously, carbon dioxide, a metabolic waste product, diffuses from the blood into the alveoli due to higher partial pressure in the blood. This exchange is vital for maintaining oxygen saturation in arterial blood while removing excess carbon dioxide, thus supporting cellular respiration and overall metabolic processes within the body.
Mechanisms of Carbon Dioxide Transport
Carbon dioxide is transported in blood using three main mechanisms: dissolved in plasma, as bicarbonate ions, and bound to hemoglobin in red blood cells.
3.1 Dissolved in Blood Plasma
A small portion of carbon dioxide, approximately 7-10%, is transported dissolved directly in blood plasma. This method allows for quick and immediate transport from tissues to the lungs. Carbon dioxide is more soluble in plasma compared to oxygen, enabling efficient diffusion. The concentration of dissolved carbon dioxide is influenced by factors such as partial pressure and temperature. When carbon dioxide is released from cells during cellular respiration, it enters the blood plasma, increasing its concentration. This dissolved carbon dioxide contributes to the regulation of blood pH and plays a role in acid-base balance. Although this transport mechanism accounts for a minor percentage, it is crucial for maintaining homeostasis and ensuring that excess carbon dioxide is removed effectively.
3.2 Buffered as Bicarbonate
Approximately 70% of carbon dioxide in the blood is transported as bicarbonate ions (HCO3-), a process crucial for maintaining acid-base balance. When carbon dioxide enters the red blood cells, it reacts with water, facilitated by the enzyme carbonic anhydrase, forming carbonic acid (H2CO3). This carbonic acid quickly dissociates into bicarbonate and hydrogen ions. The bicarbonate ions then diffuse into the plasma, while hydrogen ions help regulate blood pH. This buffering system is essential, especially during periods of increased metabolic activity, as it prevents excessive acidity, known as respiratory acidosis. Upon reaching the lungs, bicarbonate recombines with hydrogen ions to form carbon dioxide, which is then exhaled, completing the transport cycle effectively and efficiently.
3.3 Bound to Hemoglobin
About 20-23% of carbon dioxide is transported in the blood bound to hemoglobin, forming carbamino compounds. This process occurs primarily in venous blood, where carbon dioxide concentration is higher due to cellular respiration. Hemoglobin, the protein responsible for transporting oxygen, has the ability to bind with carbon dioxide at its amino acid groups, facilitating efficient transport. The binding of carbon dioxide to hemoglobin is influenced by the partial pressure of oxygen; as oxygen saturation decreases, hemoglobin’s affinity for carbon dioxide increases. This phenomenon is known as the Haldane effect and plays a significant role in promoting effective gas exchange. Upon reaching the lungs, carbon dioxide is released from hemoglobin and exhaled, contributing to respiratory health.
The Chemistry of Carbon Dioxide in Blood
The chemistry of carbon dioxide in blood involves its conversion to bicarbonate, formation of carbonic acid, and interaction with hemoglobin during gas transport and exchange.
4.1 Formation of Carbonic Acid
Carbonic acid (H2CO3) is formed in blood when carbon dioxide (CO2) reacts with water (H2O), a reaction catalyzed by the enzyme carbonic anhydrase. This reaction occurs primarily within red blood cells. When CO2 enters the bloodstream from tissues, it combines with water, producing carbonic acid, which quickly dissociates into bicarbonate (HCO3-) and hydrogen ions (H+). This process plays a vital role in maintaining acid-base balance in the body. The presence of carbonic acid influences blood pH, acting as a buffer system. When carbon dioxide levels rise, more carbonic acid is formed, leading to potential respiratory acidosis if not adequately regulated. Thus, the formation of carbonic acid is crucial for effective gas transport and overall homeostasis.
4.2 The Role of Carbonic Anhydrase
Carbonic anhydrase is a crucial enzyme that facilitates the rapid conversion of carbon dioxide and water into carbonic acid in red blood cells. This reaction is vital for efficient carbon dioxide transport in the blood. By catalyzing this process, carbonic anhydrase significantly accelerates the formation of bicarbonate ions, which account for the majority of carbon dioxide transport. In tissues, the enzyme promotes the conversion of CO2 from cellular respiration into bicarbonate, which then diffuses into the plasma. Conversely, in the lungs, carbonic anhydrase aids in the reverse reaction, allowing bicarbonate to convert back to carbon dioxide for exhalation. This dual role is essential for maintaining acid-base balance and ensuring effective gas exchange during respiration.
The Impact of Carbon Dioxide on Blood pH
Carbon dioxide levels significantly influence blood pH, affecting acid-base balance. Elevated carbon dioxide can lead to respiratory acidosis, impacting overall physiological functions in the body.
5.1 Understanding Respiratory Acidosis
Respiratory acidosis is a condition characterized by an increase in carbon dioxide levels in the blood, leading to a decrease in pH. This occurs when the respiratory system fails to adequately expel carbon dioxide, often due to conditions like chronic obstructive pulmonary disease (COPD), asthma, or respiratory failure. As carbon dioxide accumulates, it reacts with water to form carbonic acid, which dissociates into bicarbonate and hydrogen ions, lowering blood pH. Symptoms of respiratory acidosis can include confusion, lethargy, and shortness of breath. The body attempts to compensate through renal mechanisms, increasing bicarbonate reabsorption to buffer the excess hydrogen ions. Timely recognition and treatment are essential to prevent severe complications and restore acid-base balance effectively.
5.2 Acid-Base Balance in the Body
Acid-base balance is crucial for maintaining homeostasis in the body, primarily regulated by the interplay between carbon dioxide, bicarbonate, and hydrogen ions. The lungs and kidneys work together to manage blood pH effectively. When carbon dioxide levels rise, leading to increased carbonic acid formation, the pH decreases, creating an acidic environment. Conversely, when carbon dioxide is eliminated during respiration, pH increases, resulting in alkalinity. Bicarbonate acts as a vital buffer, neutralizing excess hydrogen ions and stabilizing pH levels. The body continuously adjusts breathing rates and renal function to maintain this balance. Disruptions in acid-base balance can lead to conditions such as acidosis or alkalosis, affecting overall physiological functioning and health.
The Journey of Carbon Dioxide in the Circulatory System
The journey of carbon dioxide involves its transport from tissues through venous blood to the lungs, where it is expelled during exhalation through pulmonary circulation.
6.1 From Tissues to Venous Blood
The journey of carbon dioxide begins in the tissues, where it is produced as a byproduct of cellular respiration. As cells metabolize glucose for energy, carbon dioxide accumulates in the intracellular space. Due to the concentration gradient, carbon dioxide diffuses from the cells into the surrounding interstitial fluid and then into the capillaries. Within the capillaries, carbon dioxide is transported through three primary mechanisms: dissolved in plasma, converted to bicarbonate, or bound to hemoglobin. The majority is transformed into bicarbonate ions, while some remains dissolved or bound to hemoglobin. This carbon dioxide-rich venous blood then flows through the venous system toward the heart, preparing for its journey to the lungs for elimination during respiration.
6.2 Transport through Pulmonary Circulation
Once carbon dioxide-rich venous blood reaches the heart, it is pumped into the pulmonary circulation via the right ventricle. The blood travels through the pulmonary arteries to the lungs, where gas exchange occurs in the alveoli. In this region, carbon dioxide diffuses from the blood into the alveolar spaces due to the higher concentration of carbon dioxide in the blood compared to the alveolar air. This process is facilitated by the thin walls of the alveoli and their surrounding capillaries. As carbon dioxide leaves the blood, oxygen from the inhaled air diffuses into the bloodstream, binding to hemoglobin in red blood cells. This efficient exchange ensures that carbon dioxide is expelled during exhalation, maintaining respiratory health.
Factors Influencing Carbon Dioxide Transport
Carbon dioxide transport in blood is influenced by oxygen saturation, metabolic waste production, pH levels, temperature, and overall respiratory function, affecting gas exchange efficiency.
7.1 Oxygen Saturation and Its Effects
Oxygen saturation, defined as the percentage of hemoglobin binding sites occupied by oxygen, significantly affects carbon dioxide transport in the blood. High oxygen levels increase hemoglobin’s affinity for oxygen, which can reduce its capacity to bind carbon dioxide, a phenomenon known as the Haldane effect. Conversely, low oxygen saturation enhances hemoglobin’s ability to carry carbon dioxide, facilitating efficient transport from tissues to the lungs. This relationship plays a critical role in maintaining acid-base balance and ensuring effective gas exchange. As oxygen is released in tissues, carbon dioxide is readily accepted, promoting its removal during respiration. Thus, optimal oxygen saturation levels are vital for efficient carbon dioxide transport, directly impacting respiratory and metabolic functions in the body.
7.2 Metabolic Waste and Cellular Respiration
Cellular respiration is the process by which cells convert nutrients into energy, producing carbon dioxide as a metabolic waste product. This carbon dioxide must be efficiently transported away from tissues to prevent toxicity and maintain homeostasis. During cellular respiration, glucose is metabolized in the presence of oxygen, yielding energy, water, and carbon dioxide. The carbon dioxide produced diffuses into the interstitial fluid surrounding cells and subsequently enters the bloodstream. Here, it is transported mainly as bicarbonate ions, dissolved in plasma, or bound to hemoglobin. The removal of this metabolic waste is crucial, as elevated carbon dioxide levels can lead to respiratory acidosis and disrupt acid-base balance. Thus, effective carbon dioxide transport is essential for sustaining cellular and overall physiological function.
Understanding carbon dioxide transport in blood highlights its significance for respiratory function, acid-base balance, and overall health, emphasizing the importance of efficient gas exchange processes.
8.1 Summary of Key Points
Carbon dioxide transport in the blood occurs through three primary mechanisms: dissolved in plasma, converted to bicarbonate ions, and bound to hemoglobin. The respiratory system plays a crucial role in facilitating gas exchange, where carbon dioxide is expelled from the body. Factors influencing transport include oxygen saturation, which affects hemoglobin’s ability to bind carbon dioxide, and metabolic waste production during cellular respiration. Additionally, the formation of carbonic acid and the action of carbonic anhydrase are vital for regulating pH levels. Understanding these processes is essential for recognizing how the body maintains acid-base balance and overall homeostasis. Efficient transport of carbon dioxide not only supports respiratory function but also ensures proper physiological functioning throughout the body.
8.2 Future Directions in Research
Future research on carbon dioxide transport in the blood will likely focus on enhancing our understanding of regulatory mechanisms and potential therapeutic applications. Investigating the role of transport proteins beyond hemoglobin could unveil novel pathways for carbon dioxide handling. Additionally, advancements in imaging techniques may allow for real-time monitoring of gas exchange and transport dynamics in vivo. Exploring the impact of various diseases on carbon dioxide transport mechanisms will help develop targeted interventions for conditions like chronic obstructive pulmonary disease and metabolic disorders. Furthermore, research into the influence of environmental factors, such as altitude and pollution, on carbon dioxide transport will provide insights into adaptive physiology and respiratory health. Ultimately, these studies aim to improve clinical outcomes and enhance patient care.











Post Comment